Homocysteine: Structure, Function, and Importance
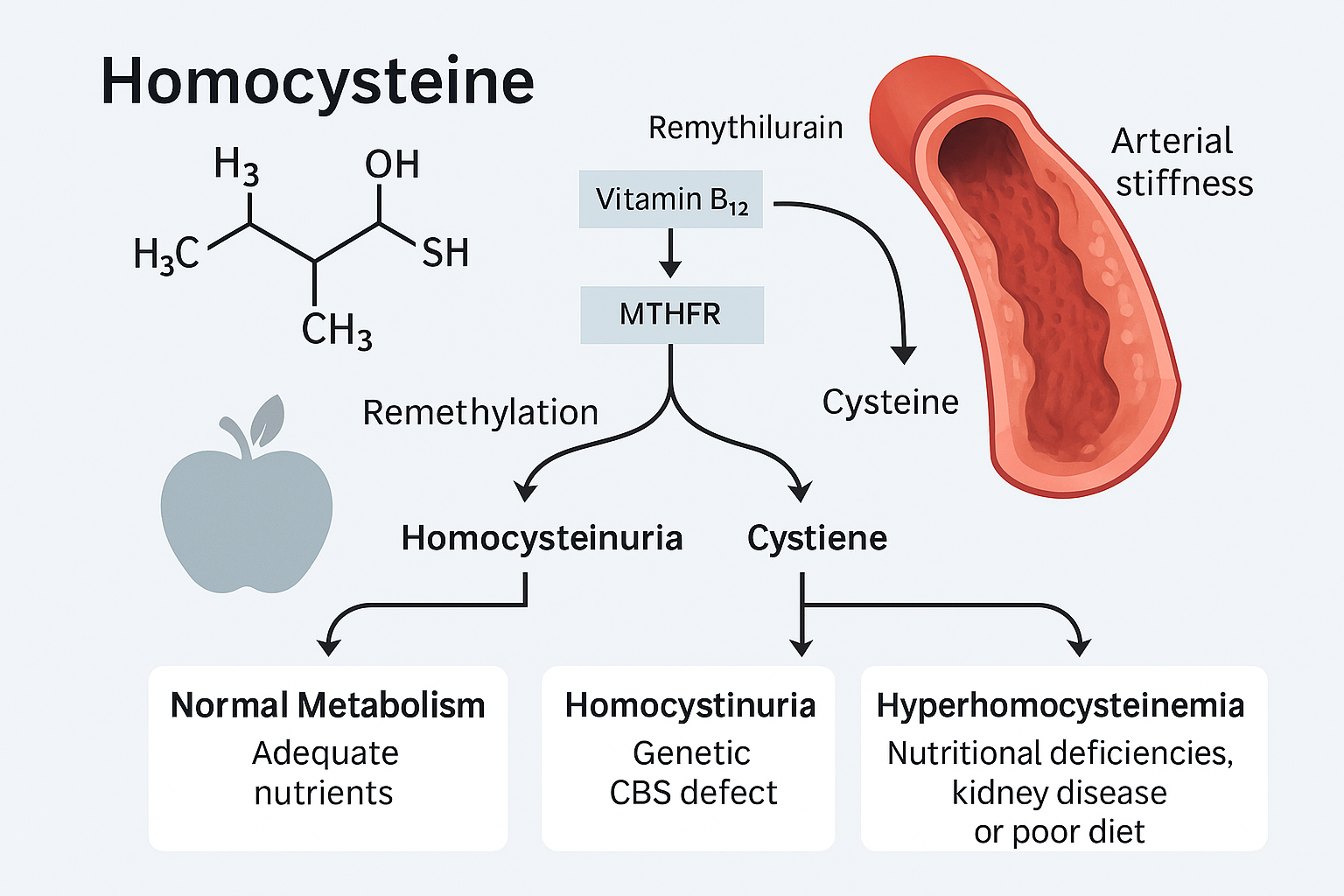
Homocysteine is a sulfur-containing amino acid that is produced in the body during the metabolism of methionine, an essential amino acid obtained from dietary protein. It is not found in the diet and does not have a specific function in protein synthesis. Instead, homocysteine serves as an intermediate in several critical biochemical pathways, particularly the methionine and folate cycles.
Under normal conditions, homocysteine is either:
1. Remethylated back into methionine using folate and vitamin B12, or
2. Converted into cysteine via the transsulfuration pathway, which requires vitamin B6.
Proper functioning of these pathways ensures that homocysteine levels remain within a healthy range.
However, disruptions in these metabolic processes—whether due to genetic mutations, nutrient deficiencies, or chronic health conditions—can lead to the accumulation of homocysteine in the blood, a condition known as hyperhomocysteinemia. Elevated homocysteine levels have been implicated in a variety of diseases, including:
– Cardiovascular diseases (e.g., atherosclerosis, thrombosis)
– Neurodegenerative disorders (e.g., Alzheimer’s disease)
– Osteoporosis
– Pregnancy complications
– Chronic kidney disease
Because of its role as a modifiable risk factor, homocysteine has become a major target in preventive medicine. Managing homocysteine through proper nutrition, supplementation, and lifestyle modifications can significantly reduce the risk of vascular and systemic complications.
Homocystinuria & Hyperhomocysteinemia: Causes, Effects, and Recovery
Introduction
Homocystinuria and hyperhomocysteinemia are disorders associated with elevated levels of homocysteine, a sulfur-containing amino acid. While homocystinuria is typically a genetic disorder, hyperhomocysteinemia can also be acquired due to lifestyle or health conditions. Both conditions pose serious risks to cardiovascular, neurological, and renal health. This document explores the differences between these conditions, their causes, symptoms, and comprehensive management strategies.
What is Homocystinuria?
Homocystinuria is a rare inherited metabolic disorder caused by the inability to properly metabolize the amino acid methionine. This leads to an accumulation of homocysteine in the blood and urine. It is usually caused by a deficiency in the enzyme cystathionine beta-synthase (CBS).
Types of Homocystinuria
1. Type I (CBS Deficiency): The classic form, resulting in high levels of both homocysteine and methionine.
2. Type II (MTHFR Deficiency): Leads to low methionine and high homocysteine due to impaired methylation.
3. Type III (Methylcobalamin Deficiency): Related to vitamin B12 metabolism, often accompanied by anemia and neuropathy.
What is Hyperhomocysteinemia?
Hyperhomocysteinemia is the medical term for elevated homocysteine levels in the blood. Unlike classic homocystinuria, it is often acquired later in life due to nutritional deficiencies, kidney disease, certain medications, or lifestyle factors.
Causes of Acquired Hyperhomocysteinemia
– Vitamin B6, B9 (folate), and B12 deficiencies
– High intake of red meat and protein without proper B-vitamin support
– Kidney dysfunction or transplant
– Use of certain medications (e.g., methotrexate, phenytoin)
– Smoking, alcohol consumption, chronic inflammation
Does It Affect Skin Color?
There is no direct evidence that homocystinuria or hyperhomocysteinemia causes changes in skin color. However, in rare cases, associated nutrient deficiencies may lead to hypopigmentation, dermatitis, or other skin issues.
Management and Recovery
Effective management involves a combination of lifestyle changes, nutritional support, and regular medical monitoring:
1. Supplementation:
– Methylcobalamin (B12): 1000–5000 mcg daily
– Methylfolate (B9): 400–800 mcg daily
– P5P (active B6): 25–50 mg daily
– Trimethylglycine (TMG): 500–1000 mg daily (optional)
– Magnesium: 300–400 mg daily
– Omega-3 (EPA/DHA): 1000–2000 mg daily
2. Diet:
– Emphasize leafy greens, beets, lentils, avocados, berries, and oily fish
– Limit red meat, sodium, alcohol, and refined sugars
3. Exercise and Lifestyle:
– Incorporate light cardio (e.g., 30-minute walks after meals)
– Use moderate-intensity resistance training
– Ensure good hydration with filtered water
– Avoid smoking and manage stress effectively
4. Monitoring:
– Regular testing of homocysteine, B-vitamin levels, and kidney function
– Cardiovascular assessments if arterial stiffness is suspected
References
Bailey, L. B., & Gregory, J. F. (1999). Folate metabolism and requirements. *The Journal of Nutrition*, 129(4), 779–782. https://doi.org/10.1093/jn/129.4.779
Refsum, H., Ueland, P. M., Nygård, O., & Vollset, S. E. (1998). Homocysteine and cardiovascular disease. *Annual Review of Medicine*, 49(1), 31–62. https://doi.org/10.1146/annurev.med.49.1.31
Mudd, S. H., Levy, H. L., & Skovby, F. (2001). Disorders of transsulfuration. In C. R. Scriver, A. L. Beaudet, W. S. Sly, & D. Valle (Eds.), *The Metabolic and Molecular Bases of Inherited Disease* (8th ed., pp. 2007–2056). McGraw-Hill.
Selhub, J. (1999). Homocysteine metabolism. *Annual Review of Nutrition*, 19(1), 217–246. https://doi.org/10.1146/annurev.nutr.19.1.217
De Bree, A., Verschuren, W. M., Kromhout, D., Kluijtmans, L. A., & Blom, H. J. (2002). Homocysteine determinants and the evidence to what extent homocysteine determines the risk of coronary heart disease. *Pharmacological Reviews*, 54(4), 599–618.
Jakubowski, H. (2008). The role of homocysteine in the pathogenesis of atherosclerosis. *Molecular Genetics and Metabolism*, 94(2), 183–189. https://doi.org/10.1016/j.ymgme.2008.01.014
National Institutes of Health. (2023). Office of Dietary Supplements: Vitamin B12. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/
National Institutes of Health. (2023). Office of Dietary Supplements: Folate. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/